2021 Materials Science Senior Design Projects
We invite you to enjoy the final presentations of the Materials Science Senior Design Projects on Friday, April 16, at 12:00 pm ET. Please contact [email protected] for the Zoom link or for more information.
A Schema for Storing Synthesis Recipes
(download poster)
Project by: Ruby Aidun
Advisor: Dr. Yevgeny Rakita, Prof. Simon J. L. Billinge
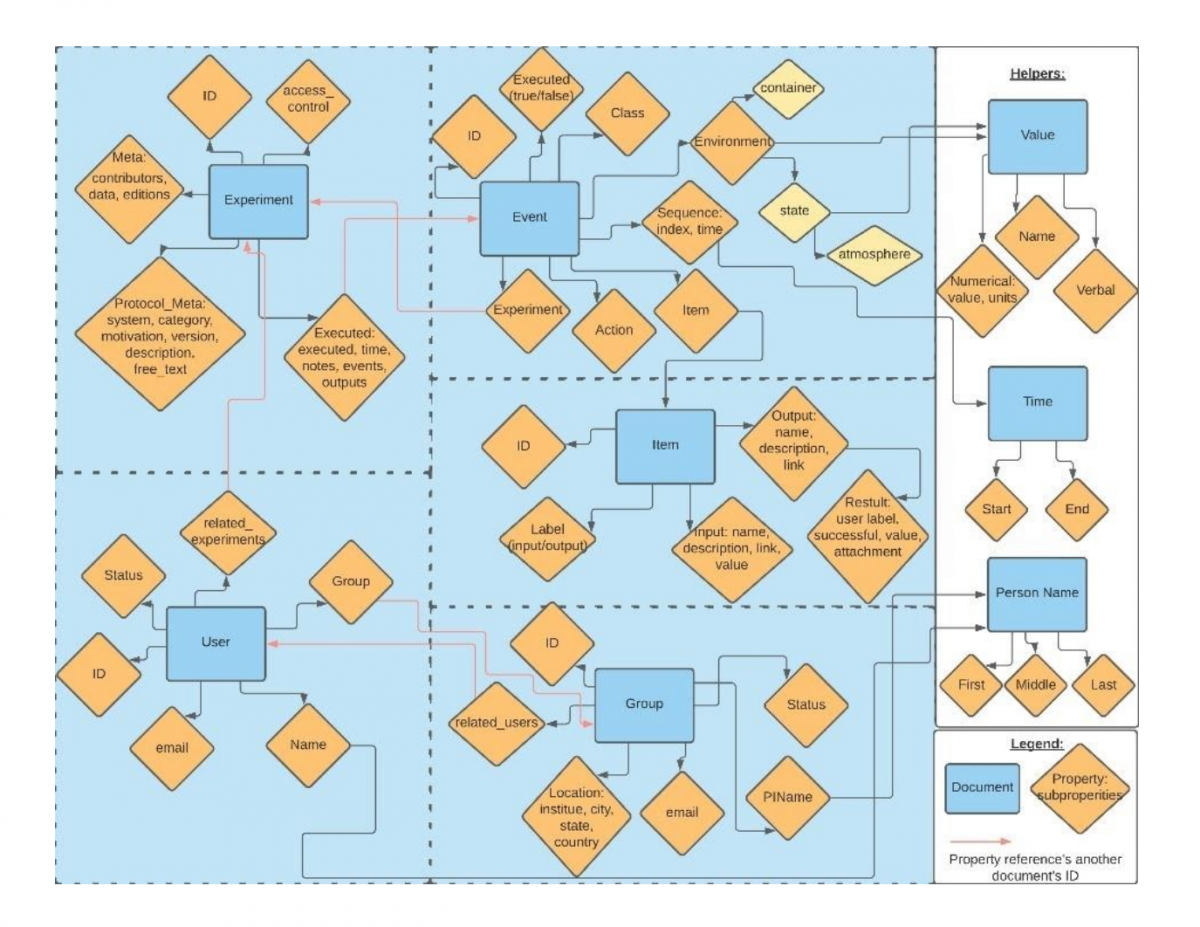
Abstract: Currently, discovering and making a new material relies on scientific intuition and time-consuming trial and error experimentation. As a result, it takes 10-20 years to bring a new material from initial research to market. Machine learning has the potential to cut down drastically the time it takes to develop novel materials. We would like to use machine learning to predict successful synthesis procedures for new materials. However, machine learning requires large amounts of data to be trained upon. Thus, a database to store experimental syntheses is needed to store this data in a machine-readable format. In this project, a schema for a non-relational database to store material synthesis procedures was developed. The schema can be found GitHub at https://github.com/GENESIS-EFRC/mosydb-schema, along with documentation at https://genesis-efrc.github.io/mosydb-schema/. We invite the materials community to try it out and provide feedback. The figure below shows an overview of the schema: the data that can be stored and the relationships between the data items.
Keywords: Materials Synthesis, Schema, Databases, Machine Learning
A Schema for Storing Synthesis Recipes
Project by: Ruby Aidun
Making Titania in the Microwave: Efficient and Better for the Environment
(download poster)
Project by: Lauren Kranis
Advisor: Prof. Simon J. L. Billinge
Abstract: Titania is a valuable nanoparticle that has a variety of future applications due to its desirable optical and electronic properties and good chemical and thermal stability. Synthesizing titania with microwave radiation is an efficient method to obtain higher crystallinity because it yields a greater percent of the desirable anatase phase at a much lower temperature than conventional synthesis techniques in a furnace. To investigate the formation products we used the pair distribution function (PDF) technique. PDF data illustrates the frequency, or the probability, of finding atoms at a distance – r – apart. We obtain the PDF by transforming x-ray diffraction data to real space via a Fourier transform. The PDF analysis on our microwave samples showed that there are at least two titania phases at the local atomic level. The phases used, for the two-phase fitting, to obtain the best goodness of fit, depend on the synthesis conditions. With a reaction temperature of 184 °C, microwave power of 840 W, and a reaction hold time of 30 minutes, a two-phase fitting of Rutile in the low r region and Anatase in the mid-range r region yielded the best goodness of fit. Previous studies show synthesis conditions with a reaction temperature of 160 °C, reaction power of 40 W, and a reaction hold time of 60 minutes resulting in a low r fitting with brookite, and mid-range r fitting with anatase. Understanding the local structure of the material and the relationship to the processing conditions will allow us to better understand how to make high quality materials optimized for their usage properties.
Keywords: PDF, Nano-particles, Titania
Making Titania in the Microwave: Efficient and Better for the Environment
Project by: Lauren Kranis
Influence of nanoCaCO3 seeding on the hydration and carbonation behavior of MgO based cements
(download poster)
Project by: Jaime Madridejos Varela
Advisor: Prof. Shiho Kawashima
Abstract: Desalination is the process of treating seawater to make clean fresh water. This process creates a harmful byproduct called brine; a sludge with ion concentrations 5 times larger than seawater as well as other chemicals from treatment. Brine is typically discharged into the sea, harming the marine life. As possible waste management solution, we can extract resources from brine that can be turned into valuable products. Mg(OH)2 can be precipitated and calcinated to be used in the production of reactive MgO cements (RMC). This material is a promising sustainable alternative to industry standard Ordinary Portland Cement (OPC), it can harden and gain strength through CO2 curing. CaCO3 can also be precipitated to be used as a nucleation seed in cement which can promote hydration and carbonation and consequently enhance its properties. Some materials used as nucleation seeds like hydromagnesite have been proven to enhance RMC properties. Others like nanoCaCO3 have only been shown to work with OPC. As we can extract CaCO3 and MgO from reject brine, this project was focused on the influence of nanoCaCO3 seeding on the hydration and carbonation of RMCs. We compared a control group composed of sand, water and MgO against a test group with a 5% replacement by weight of nanoCaCO3. We molded 2in cubes that were air cured in room conditions for the first 24 hours before carbon curing in the incubator for 28 days. We conducted isothermal calorimetry, strength testing and thermogravimetric analysis to measure and compare the kinetics and degree of hydration and carbonation. We expected the seed to produce a stronger cement as a result of improved hydration and carbonation. However, our Isothermal and TGA curves suggest little difference between both samples showing a similar degree of hydration and comparable amounts of carbonate phases. The control group also showed an average strength 18% higher than the test group. In the end, the results indicate that under these conditions nanoCaCO3 is not a good seed for RMC and showed no improvement on the resulting properties. It is possible that we could alter certain features like CO2 content, or humidity, or replacement levels to produce a cement comparable to OPC.
Keywords: Reactive Magnesium Cement, Ordinary Portland Cement, MgO, nanoCaCO3, Carbonation, Hydration, Strength, Nucleation seed.
Influence of nanoCaCO3 seeding on the hydration and carbonation behavior of MgO based cements
Project By: Jaime Madridejos Varela
Strength Enhancement of Electrode/Current Collector Interfacial Adhesion
Project by: Daye Um
Advisor: Prof. Yuan Yang
Abstract: Interfacial adhesion between electrode and current collector is important for structural stability of battery under mechanical load. Based on the peeling of results, polyacrylonitrile (PAN) binder has higher adhesion strength than Polyvinylidene fluoride (PVDF) binder, but it forms a covering on the electrode surface due to to its higher film forming property and the slow evaporation of solvent in electrolyte slurry during drying process which leads to poor electrolyte permeation and capacity loss. In this project, suitable recipes and drying processes to eliminate the covering of polyacrylonitrile (PAN) binder were found. By modification in recipes and electrode preparation process, 5% of PAN has 3 times stronger interfacial adhesion than 7.5% PVDF without trade-off battery performance.
Strength Enhancement of Electrode/Current Collector Interfacial Adhesion
Project by: Daye Um