Electrifying Chemistry
Professor Latha Venkataraman pioneers a new method for ‘green chemistry’

A series of conversations on pioneering research

Latha Venkataraman, Lawrence Gussman Professor of Applied Physics and professor of chemistry.
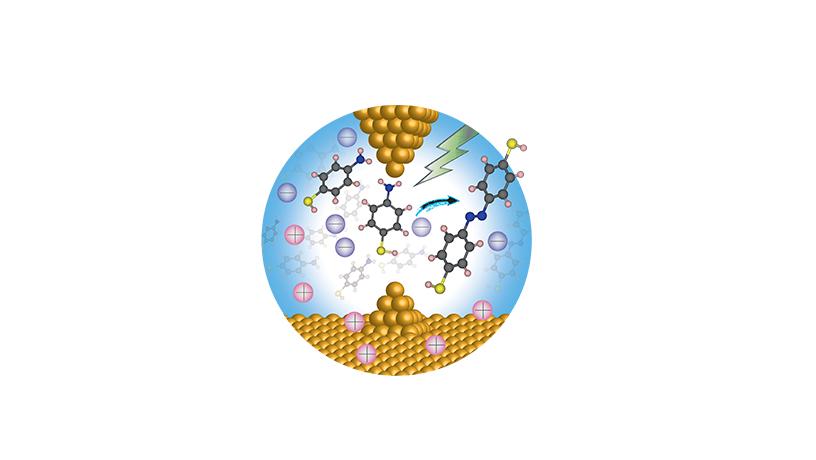
llustration of an electric field catalyzing a reaction where two molecules are coupled to form a product.
For years, applied physicist Professor Latha Venkataraman sought to create electronic circuits, each just a single molecule—research she hoped could help lay the groundwork for revolutionary advances across nanotechnology. What she didn’t expect was how this research recently uncovered a whole new way to catalyze reactions, known as electric-field-driven chemistry. Taking this breakthrough as its premise, Columbia’s new National Science Foundation Center for Chemistry with Electric Fields launched last year with Venkataraman at the helm. Together with her team, Venkataraman now seeks to turn a moment of serendipity into a host of novel techniques that could potentially transform the field by using dramatically less energy without toxic catalysts or by-products.
What’s the big idea driving your research?
My lab has traditionally been interested in understanding how current flows through molecules. In order to do that, we’ve developed experimental methods to attach single molecules to metal electrodes. A couple of years ago, we realized we were creating large electric fields at the sharp tips of the electrodes in the vicinity of the molecules and that these fields were driving chemical reactions, allowing us to study both the electronic characteristics of the reactants and the product formed. In other words, we found that the electric fields were actually doing chemistry.
Past theoretical research had predicted electric fields could drive chemical reactions: certain structures in enzymes are thought to catalyze reactions much better than other structures due to electrically charged ions organized around where the reactions occur. But no one had managed to clearly show this phenomenon occurring in a solution with an externally controlled field until our experiments. These preliminary results became the basis of our center.
This is one aspect of fundamental research that’s really fulfilling, how it opens up pathways previously unimagined. Having been trained as an experimental physicist, I never thought I would be seeking better ways to catalyze chemical reactions.
How is electric-field-driven chemistry poised to make a major impact?
There are two things we think electric fields can do: they can lower the amount of energy needed for a chemical reaction to make a product, and they drive chemical reaction selectivity, enabling formation of a specific type of product that wouldn’t be formed without the field.
Our center aims to discover how reactions can be driven by electric fields, while also figuring out the reaction mechanism itself. Once we prove that different classes of reactions can be catalyzed by electric fields, we’ll move on to figuring out how to do that at scale.
What are some recent center developments you find electrifying?
We have some preliminary results for quite a few reactions where we think electric fields have catalyzed synthesis. We’re now trying to understand exactly what products we are making and to demonstrate that it is really the field that is driving reactivity and not something else. Since we are doing these experiments with very small amounts of reactants, we yield minute amounts of products which are not trivial to characterize with standard chemical techniques. Identifying these products is like solving a puzzle, pushing the limits of our characterization tools to figure out what they are. This is really exciting.